AbstractObjective
Althoughadipocytesarethemostabundantstromalcellcomponentinbreastcancertissues,theirinteractionwithbreastcancercellshasbeenlessinvestigatedcomparedtocancer-associatedfibroblastsormacrophages.Exosomesareanovelwayofcell-cellcommunicationandhavebeendemonstratedtoplayanimportantroleinvariousbiologicalprocesses.However,toourknowledge,onlyafewstudieshavereportedtheeffectsofadipocyteexosomesontumordevelopment.Here,utilizingexosomesisolatedfrominvitromesenchymalstromal/stemcell(MSC)-differentiatedadipocytes,wesystematicallyinvestigatedthisissueinabreastcancermodel.
MaterialandmethodsExosomeswereisolatedfromMSC-differentiatedadipocytesandaddedtobreastcancercellsMCF7.CellproliferationwasdetectedbyMTS,andmigrationwasanalyzedbywoundhealingandtranswellassay.AninvivomousexenograftmodelwasusedtoevaluateMSC-differentiatedadipocyteexosomes’contributiontotumorgrowth.Signalingpathwayactivationwasevaluatedbywesternblotandimmunofluorescencestaining.
ResultsWefoundMSC-differentiatedadipocyte-derivedexosomesareactivelyincorporatedbybreastcancercellMCF7andsubsequentlypromoteMCF7proliferationandmigrationaswellasprotectMCF7fromserumderivationorchemotherapeuticdrug-inducedapoptosisinvitro.Intheinvivomousexenograftmodel,depletionofexosomesreducestumor-promotingeffectsofadipocytes.TranscriptomicanalysisofMSC-differentiatedadipocyteexosome-treatedMCF7identifiedseveralactivatedsignalingpathways,amongwhichweconfirmtheHipposignalingpathwayandfoundablockadeofthispathwayleadstoareducedgrowth-promotingeffectofadipocyteexosomes.
ConclusionTakentogether,ourfindingsprovidenewinsightsintotheroleofadipocyteexosomesinthetumormicroenvironment.
Background
Breastcancerisoneofthemostcommoncancersandthesecondleadingcauseofcancer-relatedmortalityinwomenworldwide[1].Variousfactorssuchasgeneticandepigeneticmutations,abnormalhormonelevels,andenvironmentalstimuluscontributetobreastcancerdevelopment[2,3].Emergingevidenceindicatesthatthetumormicroenvironmentplaysavitalroleincancerinitiationandprogression[4].Thetumormicroenvironmentconsistsofanumberofdifferentcomponentsincludingnon-malignantcells,surroundingbloodvessels,extracellularmatrix(ECM),andsignalingmolecules[5].Inbreastcancer,studiesfocusingoninteractionsbetweencancercellsandtumormicroenvironmenthaveemphasizedtheimportantrolesofstromalcompartmentssuchascancer-associatedfibroblastsandcancer-associatedmacrophages[6].Onecriticallyimportant,yetoftenoverlooked,tumormicroenvironmentcomponentisadipocytes,whicharethemostabundantstromalcellsinhumanbreasttissue.Increasingevidencesuggeststhatadipocytesarenotmerelyanenergy-storingcell,butcanfunctionasendocrinecellsbyproducinghormones,growthfactors,cytokines,andadipokines[7,8].Herroonetal.foundbone-trophicbreasttumorcellscouldupregulatetheoxidativestressenzymeuponexposuretoadipocyte-richenvironmentsinvitroorinvivo[9].Similarly,aconditionedmediumfromadipocyteswasreportedtoincreasemotilityofbreastcancercelllines[10].Adipocytescouldtransferfreefattyacids(FFAs)tostimulatebreastcancerinvasionviametabolicremodelingoftumorcells[11].Thesestudiessuggestthereisanintimateinteractionbetweenbreastcancercellsandadipocytes.However,theunderlyingmechanismgoverningadipocytecrosstalkwithbreastcancercellsisnotfullyunderstood.
Exosomesareanovelwayofcell-cellcommunicationandplayanimportantroleintumordevelopment[12,13,14].Adipocyte-secretedexosomeshavebeenshowntoaggravateatherosclerosisbyincreasingangiogenesis[15]andinduceinsulinresistanceinskeletalmusclethroughrepressionofPPARγ[16].Adipocytes,whicharespecializedinstoringandreleasingFFAs,areabletoshifttumormetabolismtowardtheuseofFFAsviaextracellularvesicles[17].Currently,moststudiesusemousecellline3T3-L1-differentiatedadipocytesasacellularmodel.Here,weinducedhumanadiposetissue-derivedmesenchymalstromal/stemcells(MSCs)intoadipocytes.MSCsweredefinedin2006bytheInternationalSocietyofCellularTherapy(ISCT)ascellswiththethreeproperties:(1)beadherenttoplasticunderstandardtissuecultureconditions,(2)expresscertaincellsurfaceMarkerssuchasCD73,CD90,andCD105andlackexpressionofothermarkersincludingCD45,CD34,CD14,orCD11b,CD79alpha,orCD19andHLA-DRsurfacemolecules,(3)havethecapacitytodifferentiateintoosteoblasts,adipocytes,andchondroblastsunderinvitroconditions[18].AccordingtoISCTcriteria,theisolatedMSCsareaheterogeneouspopulationofcellscontainingbothstemcellsandcellswithlowermultipotentialproperties[19].Somanyexpertsrecommendtheuseofmesenchymalstromal/stemcells(MSCs)[20,21,22].MSCs,especiallyadiposetissue-derivedMSCs,canbedifferentiatedintoadipocytesunderproperinvitrocultureconditions[23,24].Toourknowledge,onlyafewstudieshavereportedtheeffectsofadipocyteexosomesontumordevelopment.Here,utilizingexosomesisolatedfrominvitroMSC-differentiatedadipocytes,wesystematicallyinvestigatedthisissueinbreastcancer.Wefoundmesenchymalstemcell(MSC)-differentiatedadipocyteexosomescouldpromotebreastcancercellproliferationandmigrationaswellasprotectbreastcancercellsfromserumderivationorchemotherapeuticdrug-inducedapoptosisinvitro.Furthermore,exosomescontributetoinvivotumorgrowthinamousexenograftmodel.Mechanistically,theHipposignalingpathwaywasdemonstratedtobepartiallyresponsibleforthetumor-promotingeffectsofMSC-differentiatedadipocyteexosomes.Takentogether,ourfindingsprovidenewinsightsintotheroleofadipocyteexosomesinthetumormicroenvironment.
MaterialandmethodsCelllineandcultureMCF-7cellswerepurchasedfromTheCellCenteroftheChineseAcademyofMedicalSciences(Beijing,China)andculturedinDulbecco’smodifiedEagle’smediumwith4.5 g/Lglucose(H-DMEM)containing10%FBS,100 U/mlpenicillin,and100 μg/mlstreptomycin.
IsolationandexpansionofMSCsfromadulthumanadiposetissueHumanadiposetissuewasobtainedfromdonorsundergoingliposuctionaccordingtoproceduresapprovedbytheEthicsCommitteeattheChineseAcademyofMedicalSciencesandPekingUnionMedicalCollege.Theisolationandcultureprocedureforhumanadiposetissue-derivedMSCswasdescribedinourpreviouspapers[25,26].MSCsofthethirdpassagewereusedforthefollowingstudy.Ithasbeenreportedthatsenescentcellsmayproduceanti-cancerfactorsthatblockcancergrowth[27].Weperformedbeta-galactosidaseassayandfoundnosenescentcellsatpassage3.
AdipogenesisandanalysisTheculture-expandedcellsofthethirdpassageat100%confluencewereinducedinthefollowingadipogenicmediafor12 days:H-DMEMsupplementedwith10%FBS,1 μMdexamethasone,0.5 mMisobutylmethylxanthine,and1 mMascorbicacid(allreagentswerefromSigmaAldrich).Forcellstaining,cellswerestainedwithfilteredOilRedOsolution(stocksolution:3 mg/mlinisopropanol;workingsolution:60%OilRedOstocksolutionand40%distilledwater)for1 hatroomtemperatureand2 μMBODIPYstainingsolutionfor15 minat37 °C,respectively.Then,cellswerewashedwithwatertoremoveunbounddye,visualizedbymicroscopy,andphotographed.
ExosomeisolationandanalysisCellculturesusedtoisolateexosomesweregrowninserum-freeH-DMEM.Exosomeswereisolatedfromconditionedmediacollectedat48 hbyserialcentrifugationaspreviouslydescribed(Theryetal.,2006:IsolationandCharacterizationofExosomesfromCellCultureSupernatantsandBiologicalFluids),andexosomepelletswereresuspendedinPBS.ExosomeswerequantifiedbyBCAproteinquantification.Morphologyoftheexosomeswasexaminedbyelectronmicroscopyusingnegativestaining.Toexamineexosomemarkers,cellularandexosomeproteinwasextractedby10%SDSlysis.ExosomemarkersincludeCD63(Proteintech),TSG101(Proteintech),Calnexin(Proteintech),andbeta-actin(SantaCruz).ExosomesizeswereidentifiedbynanoparticletrackinganalysiswithZETAVIEW(ParticleMetrix),andtheexosomeswerediluted100–400timesin100 μLofsterilePBS.Theexosome-depletedculturemediawereobtainedafterexosomeisolationfromconditionedculturemediabyultracentrifugation.Exosomestaking-upwasinvestigatedbylabelingexosomeswithDiI(Invitrogen)andlabelingcellnucleuswithHochest33342.Dyetransferwasvisualizedbyfluorescentmicroscopy.Theco-cultureofexosomepelletsandMCF-7cellswereperformedataconcentrationof200 μg/ml.
ProliferationassayCellswereplatedin96-wellplatesatadensityof2 × 103cellsperwell.Toreducedifferenceswithinthegroup,eachgroupofcellssamplesasetoffiveparallelholes.Then,thecellswereincubatedwithanMTSreagent(CellTiter96®AQueousOneSolutionCellProliferationAssay,Promega)for2 hin37 °Cand5%CO2.Theopticaldensity(OD)valuewasmeasuredbyanELISAreader(Bio-Tek).
Scratchassay1 × 106cells/wellwereplatedina24-wellplate.Theotherday,thecellsformedamonolayer.A10-μLpipettetipwasusedtomakeastraightscratch.TheculturemediumwaschangedtoH-DMEMwhichcontainedexosomesorPBSinthesamevolume.Thecellswereincubatedina37 °Chumidifiedincubatorwith5%CO2.Thewounddistancewasmeasuredinalightmicroscopeandthetotaltimewas24 h.Allsamplesweretestedintriplicate,andthedataareexpressedasthemean ± SD.
MigrationassayBriefly,MCF-7cellswereculturedinH-DMEMwhichcontainedexosomesorPBSinthesamevolumefor48 h.Then,200 μLofcells(1 × 106/ml)suspendedinaDMEM-onlymediumwasloadedintriplicateupperchambersofthetranswellchambers(Costar)witha8-μmporesize.Anda600-μLH-DMEMmediumwith20%FBSwasaddedintothelowerchamber.Afterincubatedfor12 hina37 °Chumidifiedincubatorwith5%CO2,themigratedcellswerefixed,washed,andstainedwithcrystalvioletstainingsolution.Thenthestainingsolutionwasextractedby30%glacialaceticacid,andtheopticaldensity(OD)valuewasmeasuredbyanELISAreader(Bio-Tek).Allsamplesweretestedintriplicate,andthedataareexpressedasthemean ± SD.
ApoptosisanalysisbyflowcytometryAnnexinV-FITC/PIdoublelabelingwasusedtodeterminetheapoptosisofMCF-7cellsculturedinH-DMEMwhichcontainedexosomesorPBSinthesamevolume,aswellastheapoptosis-inducingeffectof60 μM5FU(MEC)onMCF-7cellsculturedinH-DMEMwhichcontained10%FBSwithexosomesorPBSinthesamevolume.Cellswereplatedinthe12-wellplateandtreatedthesameasabove.After48 htreatment,thecellswereharvestedbytrypsinizationandincubatedwithFITC-conjugatedAnnexinVandPIaccordingtothemanufacturer’sinstruction(BDBiosciences).TheflowcytometerBDBioscienceAccuriC6andModiFitSoftwarewereappliedforapoptosisanalysis.Atotalofatleast1 × 104cellswereanalyzedforeachsample.Allsamplesweretestedintriplicate,andthedataareexpressedasthemean ± SD.
AnimalexperimentsBALB/Cmice(5–6 weeks)werepurchasedfromtheLaboratoryAnimalCenteroftheChineseAcademyofMedicalSciences(Beijing,China).Allmicewerebredandmaintainedunderspecificpathogen-freeconditions.AnimaluseandexperimentalprocedureswereapprovedbytheAnimalCareandUseCommitteeoftheChineseAcademyofMedicalSciences.Themiceweredividedintofourgroups:onegroupreceivedasubcutaneousinjectionof2 × 106MCF-7cells.Thesecondgroupreceivedaninjectionof2 × 106MCF-7cellsand1 × 106MSC-differentiatedadipocytes.Theothergroupreceivedaninjectionof2 × 106MCF-7cellsand1 × 106MSC-differentiatedadipocytespretreatedwith20 μMGW4869for48 h.WhenMSC-differentiatedadipocyteswerepretreatedwith20 μMGW4869for48 h,theisolatedadipocyteexosomeswereundetectablewhile,inthecontrolgroup,exosomeyieldisabout100 μg/107MSC-differentiatedadipocytes.Thelastgroupreceived2 × 106MCF-7cellsand20 μMGW4869.Thetumorvolumewasmeasuredweekly.Thetumortissueswerefixedwith10%PFA.EachgroupwastreatedwithHEandKi67staining.Ki67antibodywaspurchasedfromProteintech.
Quantitativereal-timepolymerasechainreactionsCulturedcellswerelysedbyTRIzol(Invitrogen),andRNAwasextractedaccordingtothemanufacturer’sinstruction.OnemicrogramoftotalRNAfromeachsamplewasreversetranscribedusingM-MLV(Takara)inafinalvolumeof30 μL.Thepolymerasechainreaction(PCR)amplificationwascarriedoutusingtheStep-oneSystem(Bio-Rad)withSYBRGreenMastermix(Takara).Allquantitativereal-timePCR(qRT-PCR)resultswerecarriedoutinduplicateandnormalizedtoGAPDH.
WesternblottingAfterwashingtwicewithcoldPBS,cellswerelysedinRIPAlysisbuffer(Beyotime)with1 mMPMSFandproteaseinhibitorcocktailonicefor30 min,manuallyscrapedfromcultureplates,andthenquantifiedusingtheBCAProteinAssayKit(Beyotime).Proteinswereseparatedon10%sodiumdodecylsulfate–polyacrylamidegelelectrophoresis(SDS-PAGE)gels,electroblottedontoapolyvinylidenedifluoride(PVDF)membrane(0.22 μm,Millipore).Themembraneswereblockedwith5%BSAandincubatedwithspecificantibodiesovernightat4 °Candthenwereincubatedwithhorseradishperoxidase(HRP)-conjugatedsecondaryantibodyfor1 hatroomtemperature.Theprimaryantibodieswereasfollows:GAPDH(SantaCruz),YAP,p-YAP(Ser127),JAK2,p-JAK2(Tyr1007),Stat3,p-Stat3(Tyr705),SAPK/JNK,p-SAPK/JNK(Thr183/Tyr-185),P38,p-P38(Thr180/Tyr182),ERK,p-EKR1/2(Thr202/Tyr204),Akt,p-AKT(Ser473),MST1,p-MST1(Thr183)/MST2(Thr180),LATS1,p-LATS1(Ser909)(CellSignalingTechnology).AntibodyandantigencomplexesweredetectedusingachemiluminescentECLreagent(Millipore).
ImmunofluorescencestainingTheculturedcellswerefixedat4 °Cinice-coldmethanolfor10 min,washedthreetimesinphosphatebufferedsaline(PBS),andthenpermeabilizedin0.1%TritonX-100/PBSfor10 minatroomtemperature.Nonspecificbindingwasblockedwith0.5%Tween-20/PBScontaining3%bovineserumalbumin(BSA)for30 min.Theprimaryantibodies(YAPandTAZ,CellSignalingTechnology)wereincubatedat4 °Covernight.Thesecondaryantibodies(AlexaFluor488goatanti-rabbitIgG,AlexaFluor594goatanti-mouseIgG,Invitrogen)wereincubatedfor1 hatroomtemperature.TheincubatedcellswerewashedinPBS,andHoechst33342(Gibco)wasusedtovisualizethenuclei.
StatisticalanalysisDataarepresentedasmean ± SD.Fordataanalysis,weusedGraphPadPrism6.05software.ComparisonsbetweentwogroupswereanalyzedviaStudent’sttest.Comparisonsamongthreeormoregroupswereanalyzedbyaone-wayortwo-wayanalysisofvariance(ANOVA).Differenceswereconsideredstatisticallysignificantat*P 0.05and**P 0.01.
ResultsInvitrodifferentiationofadipocytesfromAD-MSCsToinvestigatetheroleofadipocyteexosomesintumordevelopment,wefirstexploredthefeasibilityofusinghumaninvitrodifferentiatedadipocytesasanewcellularmodelsincemoststudiesusemousecellline3T3-L1-differentiatedadipocytes.hAD-MSCswereculturedunderanadipogenicinductionmediumfor12 days,anddifferentiatedcellsexhibitedtypicaladipocytephenotypesasdemonstratedbymorphologyandstaining(Fig. 1a).Lipidaccumulationisanimportantindicatorofadipogenesis.TheOilRedOstainingandBODIPYstainingshowedsmallroundlipiddropletsindifferentiatedadipocytes.TheexpressionofadipocytedifferentiationmarkersincludingPPARγ,c/EBPα,HSL,aP2,LPL,AdipoQ,andFABP4wassignificantlyincreasedinMSC-differentiatedadipocytesasmeasuredbyqRT-PCR(Fig. 1b).
Fig.1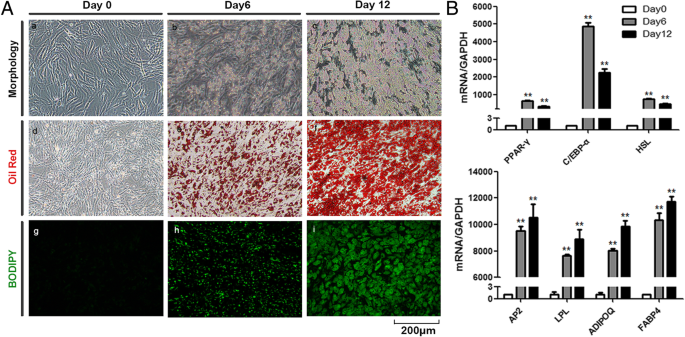
InvitrodifferentiationofadipocytesfromAD-MSCs.aMorphology,OilRedOstaining,andBODIPYstainingduringinvitroadipocytedifferentiationfromhumanAD-MSCs.bExpressionofspecificadipogenicmarkergenesanalyzedbyqRT-PCR.GAPDHwasusedasinternalcontrol(**P 0.01)
FullsizeimageCharacterizationofMSC-differentiatedadipocyteexosomesExosomesreleasedbyMSC-differentiatedadipocyteswereobservedunderatransmissionelectronmicroscopeandfoundtopresenttypicalexosomeultrastructure(Fig. 2a)anddiameterrangingfrom30to200 nm(Fig. 2b).Westernblotshowedtheabsenceofthecell-specificmarkercalnexinoractinandtheenrichmentoftheexosomalmarkerCD63andTSG101inadipocyteexosomes(Fig. 2c).AdipocyteexosomeslabeledwiththemembranedyeDilwerereadilyobservedunderafluorescentmicroscope4 hafterco-culturewithbreastcancercellMCF7andreachedpeakafter20–24 h(Fig. 2d).Together,weshowthathumaninvitrodifferentiatedadipocytessecreteexosomeswithcommonexosomalfeatures,whichareactivelytakenupbybreastcancercells.
Fig.2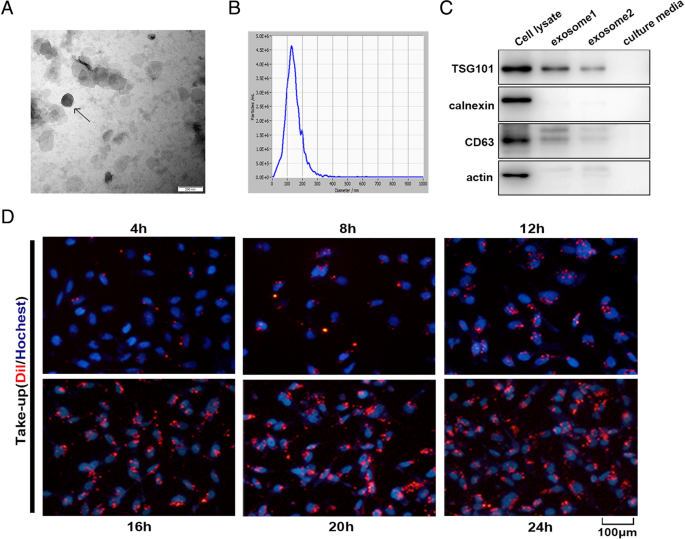
Characterizationofadipocyteexosomes.aArepresentativeelectronmicroscopyimageofadipocyteexosomes.Scalebar = 200 nm.bNTAanalysisforthenanoparticlesizedistributionofadipocyteexosomes.cWesternblotanalysisofexosomemarkerCD63,TSG101,andcell-specificmarkercalnexin.Loadedproteinforexosome1was20 μgandexosome2,10 μg.dBreastcancercellsMCF7wereincubatedwith200 μg/mLDil-labeledadipocyteexosomesfortheindicatedtimes,andinternalizationofexosomeswasdeterminedbyfluorescencemicroscopy.Scalebar = 100 μm
FullsizeimageMSC-differentiatedadipocyteexosomespromotebreastcancercellproliferationandmigrationWethenevaluatedMSC-differentiatedadipocyteexosomes’effectsonbreastcancercellproliferationandmigrationandcharacteristicabilitiesoftumordevelopment.TheproliferationrateofMCF7cellstreatedwithexosomeswassignificantlyincreasedcomparedwiththatofcontrolcellstreatedwithPBSasshowedbyMTSassay(Fig. 3a).BothwoundhealingassayandtranswellassaydemonstratedthatMCF7cellstreatedwithadipocyteexosomeshaveahighermigrationratethancontrolcellsasmanifestedbymorenumbersofmigratedcells(Fig. 3b)andfasterscratchwoundseal(Fig. 3c).Next,weassessedwhetherphysicallyremovingexosomesfromMSC-differentiatedadipocyte-conditionedmediawouldaffecttheconditionedmedium’sabilitytoincreasecellproliferationandmigration.Asexpected,comparedwiththecontrol,MCF7culturedwiththeexosome-depletedadipocyte-conditionedmediumhaveslightlylowerproliferation(Fig. 3d)andmigrationcapacityat24 h(Fig. 3e,f).
Fig.3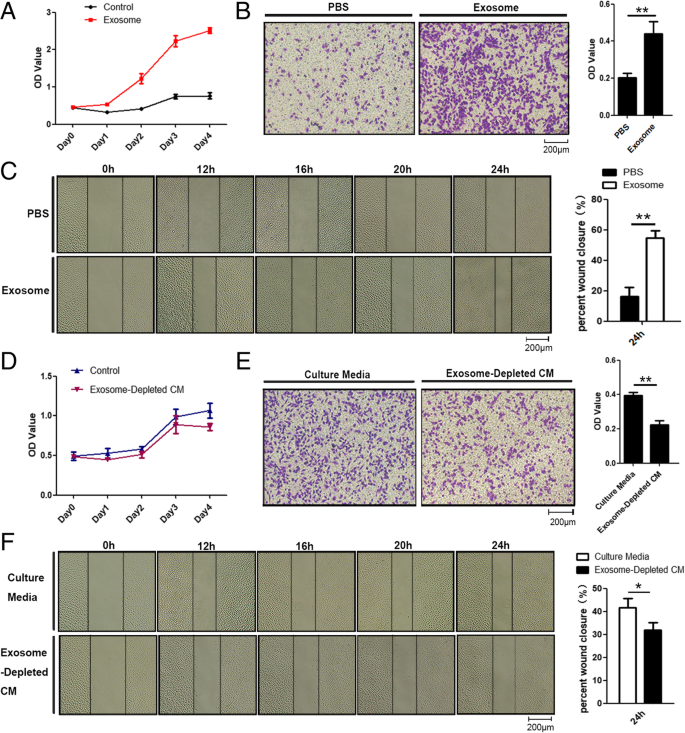
Adipocyteexosomespromotebreastcancercellproliferationandmigration.aMTSanalysisofMCF7cellstreatedwithadipocyteexosomesorPBS.bTranswellmigrationassaysshowedthatMCF7cellstreatedwithadipocyteexosomeshaveahighermigratorycapacity.Leftisarepresentativemicroscopicimageofcrystalvioletstaining.Rightshowsthestatisticalresults.cMigratoryabilityofMCF7co-culturedwithadipocyteexosomesorPBSwasdeterminedbywoundhealingassay.Leftisarepresentativemicroscopicimage.Rightshowsthestatisticalresultsat24 h.dMTSanalysisofMCF7cellstreatedwithaculturemediumorexosome-depletedculturemedium.eTranswellmigrationassaysshowedthatMCF7cellstreatedwithaculturemediumhavehighermigratorycapacitythananexosome-depletedculturemedium.Leftisarepresentativemicroscopicimageofcrystalvioletstaining.Rightshowsthestatisticalresults.fMigratoryabilityofMCF7inaculturemediumorexosome-depletedculturemediumwasdeterminedbywoundhealingassay.Leftisarepresentativemicroscopicimage.Rightshowsthestatisticalresultsat24 h
FullsizeimageMSC-differentiatedadipocyteexosomesreducebreastcancercellapoptosisAnotherhallmarkofcancercellsistheirabilitytothrivedespiteserumstarvation(SS)andchemotherapeuticdrugtreatment.Tocharacterizethepossibleeffectsofadipocyteexosomesonthishallmark,weaddedMSC-differentiatedadipocyteexosomesintoculturemediaofbreastcancercellstreatedwithSSorchemotherapeuticdrug5FU.AsshowedbyAnnexinV/PIstaining,upontreatmentwithSSfor48 h,earlyapoptoticcells(AnnexinV+/PI−)andlateapoptoticcells(AnnexinV+/PI+)weresignificantlyreducedinthepresenceofadipocyteexosomes(Fig. 4a).Similarly,adipocyteexosomesalsoreducedearlyapoptoticcellsinMCF7treatedwiththechemotherapeuticdrug5FU(60 μM)(Fig. 4b).Additionally,tomimicanSScondition,weculturedMCF7intheadipocyte-conditionedmedium(withoutserum)andexosome-depletedadipocyte-conditionedmedium(withoutserum)for48 handfoundmoreapoptoticcellswhenexosomesweredepleted(Fig. 4c).Similarly,inthepresenceofthechemotherapeuticdrug5FU,exosomedepletionleadstomoreapoptoticcells(Fig. 4d).Collectively,theseresultssuggestthatadipocyteexosomesareanimportantparticipantinregulatingbreastcancercellproliferation,migration,andapoptosis.
Fig.4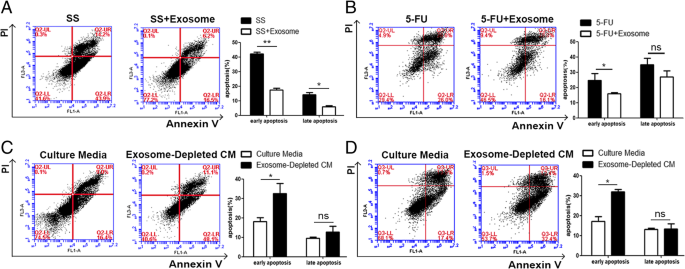
Adipocyteexosomesreducebreastcancercellapoptosis.aFlowcytometricanalysisofSS-inducedapoptosisinMCF7cellstreatedwithorwithoutadipocyteexosomes.Cellsinthelowerrightquadrant(AnnexinV+/PI−)aretheearlyapoptoticcells,andthoseintheupperrightquadrant(AnnexinV+/PI+)representlateapoptoticcells.Theexperimentswereperformedintriplicate.Therepresentativeimagesareshownattheleft,andthequantitativedataarepresentedattheright.Thedataarepresentedasthemean ± SEM(n = 3).bFlowcytometricanalysisof5FU-inducedapoptosisinMCF7cellstreatedwithorwithoutadipocyteexosomes.Therepresentativeimagesareshownattheleft,andthequantitativedataarepresentedattheright.Thedataarepresentedasthemean ± SEM(n = 3).cFlowcytometricanalysisofMCF7cellsculturedinanadipocyte-conditionedmedium(withoutserum)andexosome-depletedadipocyte-conditionedmedium(withoutserum)for48 h.Therepresentativeimagesareshownattheleft,andthequantitativedataarepresentedattheright.Thedataarepresentedasthemean ± SEM(n = 3).dFlowcytometricanalysisof5FU-inducedapoptosisinMCF7cellstreatedwithaculturemediumorexosome-depletedculturemedium.Therepresentativeimagesareshownattheleft,andthequantitativedataarepresentedattheright.Thedataarepresentedasthemean ± SEM(n = 3)
FullsizeimageMSC-differentiatedadipocyteexosomescontributetobreastcancergrowthinvivoToexplorethecontributionofadipocyteexosomesinvivo,wecarriedoutmousexenograftexperimentsbysubcutaneouslyinjectingbreastcancercellsMCF7mixedwithMatrigelaloneorwithhumanMSC-differentiatedadipocytespreviouslytreatedwithorwithoutGW4869,aninhibitorofexosomebiogenesis/release.ThepresenceofMSC-differentiatedadipocytesshowedatrendofincreasedtumorgrowthoverthe35-dayfollow-upperiodwhileblockadeofexosomegenerationwithGW4869seemedtoreducetumor-promotingeffectsofMSC-differentiatedadipocytes(Fig. 5a–d).WedeterminedtherateofcellproliferationbyIHCstainingofthetumorsectionswiththeanti-Ki67andfoundthatthenumberofKi67-positivecellswasincreasedinthepresenceofMSC-differentiatedadipocytescomparedtotheMCF7alonegroup,buttheincreasedtrendwasabolishedwhenexosomegenerationwasblocked(Fig. 5e).Similarly,thenumberofbloodvesselswasincreasedinthepresenceofadipocytesbutreducedwhenexosomegenerationwasblocked(Fig. 5f).Thus,theseresultsindicatethatadipocyteexosomescouldcontributetotumorigenesisofbreastcancercellsinvivo.
Fig.5
AdipocyteexosomescontributetohumanMCF7growthinvivo.aTumorvolumewasmeasuredandcalculatedeveryweek.b,cRepresentativephotographsoftumorsgeneratedfromnudemiceinjectedwithMCF7mixedwithMatrigelaloneorwithhumanMSC-differentiatedadipocytespreviouslytreatedwithorwithoutGW4869.dTumorweightwasmeasured35 daysaftercellinjection.eReprehensiveimagesofimmunohistochemistryanalysisofproliferationcells(ki-67positive)infrozensectionsfromtumors.fReprehensiveimagesofHEanalysisofbloodvesseldensityinfrozensectionsfromxenografts
FullsizeimageTranscriptomeanalysisofbreastcancercellstreatedwithMSC-differentiatedadipocyteexosomesWenextevaluatedthetranscriptomicalterationsinducedbyadipocyteexosomesandidentifiedactivatedsignalingpathwaysinMCF7.AdipocyteexosomescouldconvertMCF7intoatranscriptionalactivestateasdemonstratedbymoreupregulatedgenes(Fig. 6a).Unsupervisedclusteringidentifiedupregulationofgenesignaturesrelatedtocellproliferation,programmedcelldeath,migration,andangiogenesisinadipocyteexosome-treatedMCF7(Fig. 6b–e).qRT-PCRanalysisconfirmedtheincreasedexpressionofselectedgenesfromtheabovementionedgenesignatures(Fig. 6g–j).Interestingly,KEGGanalysisidentifiedatleast20signalingpathwayswithknownfunctionsintumordevelopment(Fig. 6f).
Fig.6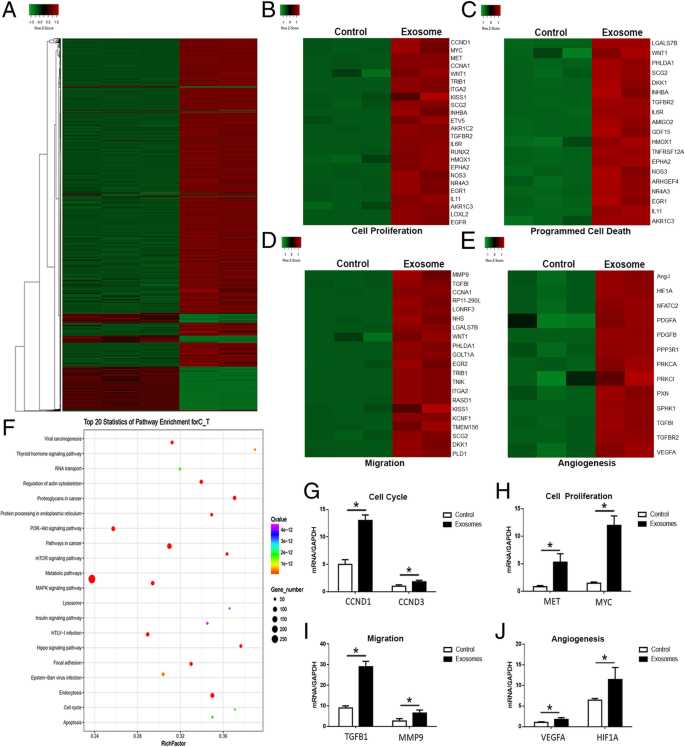
Transcriptomeanalysisofbreastcancercellstreatedwithadipocyteexosomes.aHeatmapshoweddifferentiallyexpressedgenesinadipocyteexosome-treatedMCF7andcontrolMCF7.b–eUpregulationofcancerphenotype-associatedgenesinadipocyteexosome-treatedMCF7.bGenesassociatedwithcellproliferation.cGenesassociatedwithprogrammedcelldeath.dGenesassociatedwithmigration.eGenesassociatedwithangiogenesis.fTop20signalingpathwaysenrichedinadipocyteexosome-treatedMCF7.g–jqRT-PCRconfirmedtheincreasedexpressionofselectedgenesassociatedwithcellcycle(G,CCND1,CCND3),cellproliferation(h,MET,MYC),migration(i,TGFB1,MMP9),andangiogenesis(j,VEGFA,HIF1A)
FullsizeimageMSC-differentiatedadipocyteexosomesactivatedtheHipposignalingpathwayinbreastcancercellsAmongthem,wechosePI3K-Akt,MAPK,Hippo,andJAK-STATforfurtheranalysis.WesternblotconfirmedthephosphorylationofJAK,JNK,ERK,andP38aswellasthedephosphorylationofYAP(Fig. 7a).Thecorrespondingpathwayinhibitorsalteredsuchaphosphorylationstatus(Fig. 7b–e)andattenuatedthetumorgrowth-promotingeffectofadipocyteexosomes,withHippoinhibitionexhibitingthemostsignificanteffect(Fig. 7f).Specifically,Fig. 4hdemonstratestheincreasedphosphorylationofYAPupstreamkinaseMST1.Figure 7g,ishowsthetransportationofYAP/TAZ(twokeytranscriptionfactorsoftheHipposignalingpathway)intothenucleuswheretheyactivatetranscriptionofthedownstreamgenessuchasCTGF,ANKDR1,andCYR61(Fig. 7j).Collectively,theseresultsshowedthatadipocyteexosomesactivatedtheHipposignalingpathwayintheMCF7cells.
Fig.7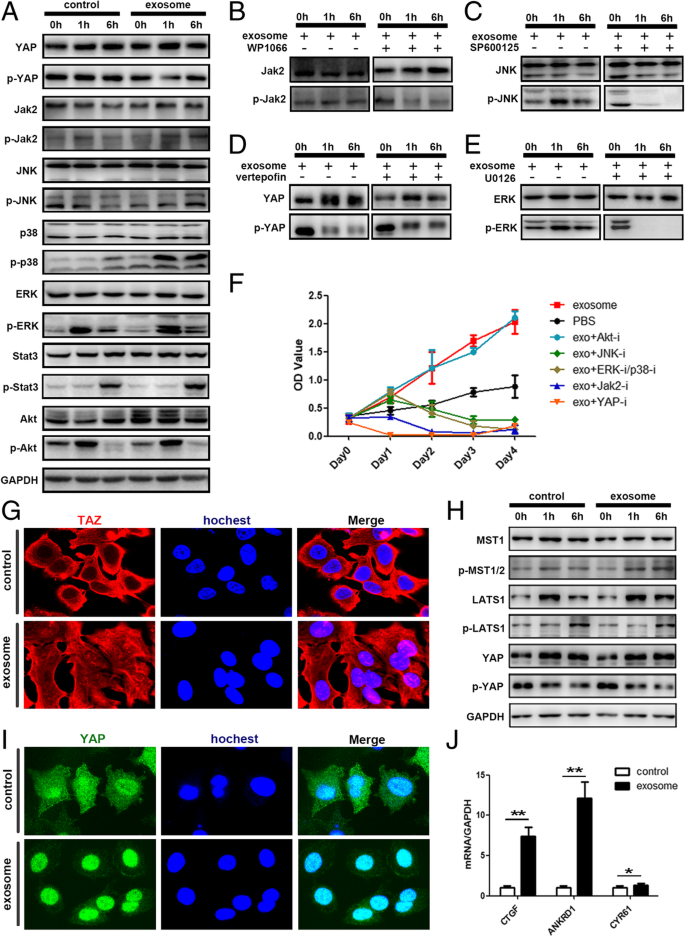
AdipocyteexosomesactivatedtheHipposignalingpathwayinMCF7cells.aPhosphorylationofYAP,Jak2,JNK,P38,ERK,Stat3,andAKTwasanalyzedbywesternblot.GAPDHwasusedastheloadingcontrol.b–ePhosphorylationofkeyproteinswasanalyzedafteradditionofspecificsignalingpathwayinhibitor.bWP1066inhibitorofJAK2/STAT3.cSP600125inhibitorofJNK.dVerteporfininhibitorofHippo.eU0126inhibitorofERK/P38.fMTSanalysisofMCF7cellstreatedwithadipocyteexosomesorPBSinthepresenceorabsenceofdifferentsignalingpathwayinhibitors.gRepresentativeimmunofluorescencestainingimagesofnucleartranslocationofTAZinadipocyteexosome-treatedMCF7.hPhosphorylationofkeyproteinsoftheHipposignalingpathway.iRepresentativeimmunofluorescencestainingimagesofnucleartranslocationofYAPinadipocyteexosome-treatedMCF7.jExpressionofYAP/TAZdownstreamgenes.GAPDHwasusedasaninternalcontrol
FullsizeimageDiscussionNumerousstudieshavedemonstratedthatthetumormicroenvironmentcouldcooperatetomodulatemalignantbehaviorsoftumorscells[5,28,29].Inbreastcancer,thecellularcomponentsoftumormicroenvironmentsincluderesidentfibroblasts,adipocytes,anumberofrecruitedimmunecells,andnewlyformedbloodvesselswiththeirassociatedcells[30].Dynamicandreciprocalcommunicationbetweentumorcellsandsurroundingcompartmentshasbeenintensivelyinvestigated.However,inthiscontext,verylittleattentionhasbeengiventoadipocytes,althoughtheyrepresentthemostprominentcelltypeinbreasttumormicroenvironment.Traditionally,adipocytesarethoughttofunctionasenergystoragecells.Now,accumulatingevidencesuggeststhattheycouldalsoserveasendocrinecellsbysecretingadipokines[31].Here,wechoseMSC-differentiatedadipocytesasacellularmodeltostudyinteractionsbetweenadipocytesandbreastcancercells.TheadipocytesaredifferentiatedbyculturinghumanMSCsunderadipogenicconditionsandarefullycharacterizedbymorphology,staining,andmarkergeneexpression.Wefoundthetumor-promotingeffectsofMSC-differentiatedadipocyteswerereducedwhenadipocyteexosomesweredepleted.
Inrecentyears,muchinteresthasbeendevotedtoexosomes,whichfunctionascarriersofbioactiveproteins,lipids,andnuclearacidsandareincreasinglyregardedascrucialplayersincell-cellcommunications[32,33].Inthepresentstudy,wecharacterizedexosomessecretedbyinvitrodifferentiatedhumanadipocytes.TheuptakeoftheseexosomesbybreastcancercellsMCF7wasobservedbyimmunofluorescencestaining,confirmingdirectinteractionbetweenadipocyteexosomesandcancercells.Adipocyteexosometreatmentbringsaboutsustainedchangesintheproliferationandanti-apoptosisofbreastcancercells.Toourknowledge,thisisthefirstreportedabouttheeffectsofMSC-differentiatedadipocyteexosomesonbreastcancercells.Previousstudieshaveexploredtheroleofadipocyteexosomesininflammationandinsulinresistance.Forexample,adipocyteexosomescoulddifferentiatemonocytesintomacrophagescharacteristicofhumanadiposetissuemacrophages(ATM),definedbyreleaseofbothpro-andanti-inflammatorycytokines[34].Wangetal.foundadipocyteexosomescouldaggravateatherosclerosisbyincreasingvasavasorumangiogenesisindiabeticApoE−/−mice[15]whileadipocyteexosomesinduceinsulinresistanceinskeletalmuscle[16].Adipocyteexosomeshavealsobeenreportedtopromotethegrowthofhepatocellularcarcinomabytargetingdeubiquitination-relatedUSP7[35]andpromotemelanomaaggressivenessthroughfattyacidoxidation[36].Here,ourstudyaddedanewfunctionofadipocyteexosomesinbreastcancerregulation.Currently,itremainslargelyunknownhowadipocytesinfluencebreasttumorcellbehaviororwhetheranyoftheparacrinefactorssecretedbyadipocytescausechangesinthephenotypicbehaviorofthemalignantcells.Ourresultsprovidenewinsightsintoexosomeswhichareemergingasanovelwayofcell-cellcommunication.
Hipposignalingisoneofthemajorpathwayscontrollingtumorigenesis.KeycomponentsoftheHippopathwayregulatebreasttumorgrowth,metastasis,anddrugresistance[37].Here,wefoundadipocyteexosomescouldactivatetwokeydownstreameffectorproteinsofHippo,theYAPandTAZ,asdemonstratedbyFig. 7.
Takentogether,wefoundthatinvitrohAD-MSC-differentiatedadipocytescouldsecreteexosomeswhichareactivelytakenupbybreastcancercelllineMCF7andsubsequentlypromotebreastcancercellMCF7proliferationandmigrationaswellasprotectMCF7fromserumderivationorchemotherapeuticdrug-inducedapoptosisinvitro.Intheinvivomousexenograftmodel,depletionofexosomesreducedtumor-promotingeffectsofadipocyteswhichimpliesthecontributionofadipocyteexosomesinthetumormicroenvironment.Furthermore,wefoundtheHipposignalingpathwaywaspartiallyresponsibleforthetumor-promotingeffectsofadipocyteexosomes.Ourdatasuggestthatadipocyteexosomescouldactasanadditionalmechanismcontributingtobreasttumormicroenvironmentandmayofferanoveltherapeuticmodalitytotargetbreastcancergrowth.However,ourstudyhasalimitation.TheinvivomousexenograftexperimentwasperformedusingadipocytestreatedwithGW4869whileourinvitrostudiesweredonewithpureexosomes.Morestudiesareneededtofurtherexploretheinvivoroleofadipocyteexosomes.
ConclusionsCollectively,ourdataindicatedthat(i)adipocyteexosomescouldbeactivelyincorporatedintobreastcancercellsandsignificantlychangedtranscriptome,particularlygenesassociatedwithtumordevelopment,(ii)depletionofexosomesfromadipocytereducedtumor-promotingeffectsofadipocytes,and(iii)theHipposignalingpathwaywasactivatedinadipocyteexosomeswhichtreatedbreastcancercells.Ourresultsprovidednewinsightsintotheroleofadipocyteexosomesinthebreasttumormicroenvironment.
Quantitativereal-timePCR
References1.DeSantisCE,MaJ,GodingSauerA,NewmanLA,JemalA.Breastcancerstatistics,2017,racialdisparityinmortalitybystate.CACancerJClin.2017;67(6):439–48.
ArticleGoogleScholar2.MarcotteR,SayadA,BrownKR,Sanchez-GarciaF,ReimandJ,HaiderM,VirtanenC,BradnerJE,BaderGD,MillsGB,etal.Functionalgenomiclandscapeofhumanbreastcancerdrivers,vulnerabilities,andresistance.Cell.2016;164(1–2):293–309.
CASArticleGoogleScholar3.LanzinoM,MarisP,SirianniR,BaroneI,CasaburiI,ChimentoA,GiordanoC,MorelliC,SisciD,RizzaP,etal.DAX-1,asanandrogen-targetgene,inhibitsaromataseexpression:anovelmechanismblockingestrogen-dependentbreastcancercellproliferation.CellDeathDis.2013;4:e724.
CASArticleGoogleScholar4.ChengCJ,BahalR,BabarIA,PincusZ,BarreraF,LiuC,SvoronosA,BraddockDT,GlazerPM,EngelmanDM,etal.MicroRNAsilencingforcancertherapytargetedtothetumourmicroenvironment.Nature.2015;518(7537):107–10.
CASArticleGoogleScholar5.HuiL,ChenY.Tumormicroenvironment:sanctuaryofthedevil.CancerLett.2015;368(1):7–13.
CASArticleGoogleScholar6.OrimoA,GuptaPB,SgroiDC,Arenzana-SeisdedosF,DelaunayT,NaeemR,CareyVJ,RichardsonAL,WeinbergRA.StromalfibroblastspresentininvasivehumanbreastcarcinomaspromotetumorgrowthandangiogenesisthroughelevatedSDF-1/CXCL12secretion.Cell.2005;121(3):335–48.
CASArticleGoogleScholar7.CozzoAJ,FullerAM,MakowskiL.Contributionofadiposetissuetodevelopmentofcancer.ComprPhysiol.2017;8(1):237–82.
ArticleGoogleScholar8.StrongAL,BurowME,GimbleJM,BunnellBA.Concisereview:theobesitycancerparadigm:explorationoftheinteractionsandcrosstalkwithadiposestemcells.StemCells.2015;33(2):318–26.
CASArticleGoogleScholar9.HerroonMK,RajagurubandaraE,DiedrichJD,HeathEI,PodgorskiI.Adipocyte-activatedoxidativeandERstresspathwayspromotetumorsurvivalinboneviaupregulationofHemeOxygenase1andSurvivin.SciRep.2018;8(1):40.
ArticleGoogleScholar10.CarterJC,ChurchFC.Maturebreastadipocytespromotebreastcancercellmotility.ExpMolPathol.2012;92(3):312–7.
CASArticleGoogleScholar11.WangYY,AttaneC,MilhasD,DiratB,DauvillierS,GuerardA,GilhodesJ,LazarI,AletN,LaurentV,etal.Mammaryadipocytesstimulatebreastcancerinvasionthroughmetabolicremodelingoftumorcells.JCIInsight.2017;2(4):e87489.
ArticleGoogleScholar12.SundararajanV,SarkarFH,RamasamyTS.Theversatileroleofexosomesincancerprogression:diagnosticandtherapeuticimplications.CellOncol(Dordr).2018;41(3):223–52.
CASArticleGoogleScholar13.HiguchiH,YamakawaN,ImadomeKI,YahataT,KotakiR,OgataJ,KakizakiM,FujitaK,LuJ,YokoyamaK,etal.RoleofexosomesasaproinflammatorymediatorinthedevelopmentofEBV-associatedlymphoma.Blood.2018;131(23):2552–67.
CASArticleGoogleScholar14.LinLY,YangL,ZengQ,WangL,ChenML,ZhaoZH,YeGD,LuoQC,LvPY,GuoQW,etal.Tumor-originatedexosomallncUEGC1asacirculatingbiomarkerforearly-stagegastriccancer.MolCancer.2018;17(1):84.
ArticleGoogleScholar15.WangF,ChenFF,ShangYY,LiY,WangZH,HanL,LiYH,ZhangL,TiY,ZhangW,etal.Insulinresistanceadipocyte-derivedexosomesaggravateatherosclerosisbyincreasingvasavasorumangiogenesisindiabeticApoE(-/-)mice.IntJCardiol.2018;265:181–7.
ArticleGoogleScholar16.YuY,DuH,WeiS,FengL,LiJ,YaoF,ZhangM,HatchGM,ChenL.Adipocyte-derivedexosomalMiR-27ainducesinsulinresistanceinskeletalmusclethroughrepressionofPPARgamma.Theranostics.2018;8(8):2171–88.
CASArticleGoogleScholar17.LazarI,ClementE,AttaneC,MullerC,NietoL.Anewroleforextracellularvesicles:howsmallvesiclescanfeedtumors’bigappetite.JLipidRes.2018;59(10):1793–804.
CASArticleGoogleScholar18.DominiciM,LeBlancK,MuellerI,Slaper-CortenbachI,MariniF,KrauseD,DeansR,KeatingA,ProckopD,HorwitzE.Minimalcriteriafordefiningmultipotentmesenchymalstromalcells.TheInternationalSocietyforCellularTherapypositionstatement.Cytotherapy.2006;8(4):315–7.
CASArticleGoogleScholar19.SquillaroT,PelusoG,GalderisiU.Clinicaltrialswithmesenchymalstemcells:anupdate.CellTransplant.2016;25(5):829–48.
ArticleGoogleScholar20.SensebeL,GadelorgeM,Fleury-CappellessoS.Productionofmesenchymalstromal/stemcellsaccordingtogoodmanufacturingpractices:areview.StemCellResTher.2013;4(3):66.
CASArticleGoogleScholar21.LaroyeC,GibotS,ReppelL,BensoussanD.Concisereview:mesenchymalstromal/stemcells:anewtreatmentforsepsisandsepticshock?StemCells.2017;35(12):2331–9.
ArticleGoogleScholar22.MatthayMA,PatiS,LeeJW.Concisereview:mesenchymalstem(stromal)cells:biologyandpreclinicalevidencefortherapeuticpotentialfororgandysfunctionfollowingtraumaorsepsis.StemCells.2017;35(2):316–24.
ArticleGoogleScholar23.YuanC,ChakrabortyS,ChittaKK,SubramanianS,LimTE,HanW,BhanuPrakashKN,SugiiS.Fastadipogenesistrackingsystem(FATS)-arobust,high-throughput,automation-readyadipogenesisquantificationtechnique.StemCellResTher.2019;10(1):38.
ArticleGoogleScholar24.ParkJS,KimM,SongNJ,KimJH,SeoD,LeeJH,JungSM,LeeJY,LeeJ,LeeYS,etal.AreciprocalroleoftheSmad4-Tazaxisinosteogenesisandadipogenesisofmesenchymalstemcells.StemCells.2018;37(3):368–81.
ArticleGoogleScholar25.FuY,DengJ,JiangQ,WangY,ZhangY,YaoY,ChengF,ChenX,XuF,HuangM,etal.Rapidgenerationoffunctionalhepatocyte-likecellsfromhumanadipose-derivedstemcells.StemCellResTher.2016;7(1):105.
ArticleGoogleScholar26.LiangX,ZhangL,WangS,HanQ,ZhaoRC.ExosomessecretedbymesenchymalstemcellspromoteendothelialcellangiogenesisbytransferringmiR-125a.JCellSci.2016;129(11):2182–9.
CASArticleGoogleScholar27.OzcanS,AlessioN,AcarMB,ToprakG,GonenZB,PelusoG,GalderisiU.Myelomacellscancorruptsenescentmesenchymalstromalcellsandimpairtheiranti-tumoractivity.Oncotarget.2015;6(37):39482–92.
PubMedPubMedCentralGoogleScholar28.TangYA,ChenYF,BaoY,MaharaS,YatimS,OguzG,LeePL,FengM,CaiY,TanEY,etal.HypoxictumormicroenvironmentactivatesGLI2viaHIF-1alphaandTGF-beta2topromotechemoresistanceincolorectalcancer.ProcNatlAcadSciUSA.2018;115(26):E5990–9.
CASArticleGoogleScholar29.WangT,LuR,KapurP,JaiswalBS,HannanR,ZhangZ,PedrosaI,LukeJJ,ZhangH,GoldsteinLD,etal.Anempiricalapproachleveragingtumorgraftstodissectthetumormicroenvironmentinrenalcellcarcinomaidentifiesmissinglinktoprognosticinflammatoryfactors.CancerDiscov.2018;8(9):1142–55.
CASArticleGoogleScholar30.SoysalSD,TzankovA,MuenstSE.Roleofthetumormicroenvironmentinbreastcancer.Pathobiol.2015;82(3–4):142–52.
CASArticleGoogleScholar31.ChkourkoGuskyH,DiedrichJ,MacDougaldOA,PodgorskiI.Omentumandbonemarrow:howadipocyte-richorganscreatetumourmicroenvironmentsconduciveformetastaticprogression.ObesRev.2016;17(11):1015–29.
CASArticleGoogleScholar32.TaiYL,ChenKC,HsiehJT,ShenTL.Exosomesincancerdevelopmentandclinicalapplications.CancerSci.2018;109(8):2364–74.
CASArticleGoogleScholar33.RuivoCF,AdemB,SilvaM,MeloSA.Thebiologyofcancerexosomes:insightsandnewperspectives.CancerRes.2017;77(23):6480–8.
CASArticleGoogleScholar34.KranendonkME,VisserenFL,vanBalkomBW,Nolte-‘tHoenEN,vanHerwaardenJA,deJagerW,SchipperHS,BrenkmanAB,VerhaarMC,WaubenMH,etal.Humanadipocyteextracellularvesiclesinreciprocalsignalingbetweenadipocytesandmacrophages.Obesity(SilverSpring).2014;22(5):1296–308.
CASArticleGoogleScholar35.ZhangH,DengT,GeS,LiuY,BaiM,ZhuK,FanQ,LiJ,NingT,TianF,etal.ExosomecircRNAsecretedfromadipocytespromotesthegrowthofhepatocellularcarcinomabytargetingdeubiquitination-relatedUSP7.Oncogene.2018.https://doi.org/10.1038/s41388-018-0619-z.[Epubaheadofprint].
36.LazarI,ClementE,DauvillierS,MilhasD,Ducoux-PetitM,LeGonidecS,MoroC,SoldanV,DalleS,BalorS,etal.Adipocyteexosomespromotemelanomaaggressivenessthroughfattyacidoxidation:anovelmechanismlinkingobesityandcancer.CancerRes.2016;76(14):4051–7.
CASArticleGoogleScholar37.ZhouX,WangS,WangZ,FengX,LiuP,LvXB,LiF,YuFX,SunY,YuanH,etal.EstrogenregulatesHipposignalingviaGPERinbreastcancer.JClinInvest.2015;125(5):2123–35.
ArticleGoogleScholarDownloadreferences
AcknowledgementsNotapplicable.
FundingThisstudywassupportedbyCAMSInnovationFundforMedicalSciences(2017-I2M-3-007),TheNationalKeyResearchandDevelopmentProgramofChina(2016YFA0101000,2016YFA0101003),BeijingKeyLaboratoryofNewDrugDevelopmentandClinicalTrialofStemCellTherapy(BZ0381).
AvailabilityofdataandmaterialsThedatasetsusedand/oranalyzedduringthecurrentstudyareavailablefromthecorrespondingauthoronreasonablerequest.
AuthorinformationAuthornotesShihuaWangandXiaodongSucontributedequallytothiswork.
AffiliationsInstituteofBasicMedicalSciencesChineseAcademyofMedicalSciences,SchoolofBasicMedicinePekingUnionMedicalCollege,CenterofExcellenceinTissueEngineeringChineseAcademyofMedicalSciences,Beijing,100005,ChinaShihuaWang, XiaodongSu, MeiqianXu, XianXiao, HonglingLi RobertChunhuaZhaoBrainTumorResearchCenter,BeijingNeurosurgicalInstitute,BeijingTiantanHospitalaffiliatedtoCapitalMedicalUniversity,TiantanXili6,DongchengDistrict,Beijing,100050,ChinaXiaodongSuDepartmentofGeneticsandCellBiology,BasicMedicalCollege,QingdaoUniversity,308NingxiaRoad,Qingdao,266071,ChinaXiaoxiaLiCellTherapyProgram,PrincessMargaretHospital,Toronto,Ontario,M5G2M9,CanadaArmandKeatingInstituteofBiomaterialsandBiomedicalEngineering,UniversityofToronto,Toronto,Ontario,M5G2M9,CanadaArmandKeatingInstituteofMedicalScience,UniversityofToronto,Toronto,Ontario,M5G2M9,CanadaArmandKeatingAuthorsShihuaWangViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarXiaodongSuViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarMeiqianXuViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarXianXiaoViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarXiaoxiaLiViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarHonglingLiViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarArmandKeatingViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarRobertChunhuaZhaoViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarContributionsSHWdesignedandanalyzedtheexperimentsandwrotethemanuscript.XDSperformedandanalyzedtheexperimentsandpreparedthefigures.MQX,XX,andXXLperformedtheexperiments.HLL,AK,andRCHZguidedtheexperiment.Allauthorshavereadandapprovedthefinalmanuscript.
CorrespondingauthorsCorrespondencetoHonglingLiorArmandKeatingorRobertChunhuaZhao.
EthicsdeclarationsEthicsapprovalandconsenttoparticipateNotapplicable.







